This review examines the emerging potential of CAR-engineered natural killer (CAR-NK) cells to transform cancer immunotherapy. While CAR-T therapies have achieved notable success in certain haematological malignancies, their efficacy in solid tumours remains limited. Furthermore, NK cells can be delivered as readily available non-self “off-the-shelf” products without the risk of graft versus host disease that accompanies equivalent T cells. We highlight the key challenges posed by the tumour microenvironment – including hypoxia, immunosuppression, metabolic stress, and antigen heterogeneity – how these factors impair NK cell function, and the latest engineering strategies and combinatorial approaches aimed at enhancing CAR-NK activity and accelerating clinical translation in solid tumours.
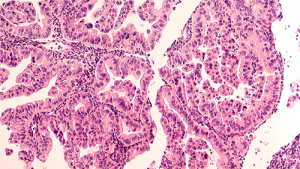
The challenge: T cell malignancies represent a broad, highly heterogeneous subset of lymphomas with poor prognosis. Chimeric antigen receptor (CAR)-T cell therapy holds great promise in treating B cell lymphoma and multiple myeloma. However, understanding its efficacy in treating T cell lymphoma remains challenging, primarily due to the lack of tumor-specific targets and intra-tumor heterogeneity.
The solution: In this proof-of-concept study, we developed and characterized three distinct CAR-T cells targeting tumor-associated glycoprotein 72 (TAG-72), C-C chemokine receptor type 4 (CCR4) and tumor necrosis factor receptor CD30 respectively and assessed their anti-tumor efficacy against T cell malignancies in vitro.
Key outcomes: These findings define TAG-72 and CD30-targeting CAR-T cells as a promising strategy against T cell malignancies and highlight the potential of dual or combination CAR-T cell therapies for this aggressive disease.
The challenge: Chimeric antigen receptor (CAR)-T cells have shown significant clinical success in treating chemotherapy-resistant or refractory ‘liquid tumors’ such as B cell malignancies. In contrast, positive outcomes in solid tumor settings remains limited. Novel approaches are required to enhance CAR-T cell efficacy in the heterogenous, immunosuppressive tumor microenvironment.
The solution: We asked whether CRISPR-Cas9 deletion of T cell-inhibitory enzymes enhance CAR-T cell efficacy against solid tumors such as ovarian cancer.
Key outcomes: We demonstrate CRISPR-engineered deletion of two key immune-suppressor genes in TAG-72 targeting CAR-T cells greatly improves their ability to eliminate ovarian cancer in mice. The enduring response potentially overcomes challenges of tumor heterogeneity and immune suppression. This study highlights advances in CAR-T cell efficacy for treating solid tumors.
The challenge: Cutaneous T cell lymphoma (CTCL) is a heterogenous disease that presents with a variety of clinical features which involves both the blood and skin. The patients are considered incurable and have limited treatment options with high likelihood of disease relapse. Taken together, this highlights a need for novel and more effective therapies.
The solution: Chimeric antigen receptor (CAR) immune cell therapy is a new pillar of cancer treatment that has demonstrated clear, and enduring, benefits clinically in other blood-based cancer indications. We asked whether a CAR-based approach would deliver a more effective treatment option for CTCL patients.
Key outcomes: This study shows for the first time, that tumour-associated glycoprotein-72 (TAG-72) is expressed on disease T cells in CTCL patients and that these cells can be eradicated with TAG-72 targeting CAR-T cells. Our preclinical studies outline that TAG-72 may be a novel target for CAR-T cells therapy for CTCL.
The challenge: Manufacture of chimeric antigen receptor (CAR)-T cells usually involves the use of viral delivery systems to achieve high transgene expression. However, it can be costly and may result in random integration of the CAR into the genome, creating several disadvantages including variation in transgene expression, functional gene silencing and potential oncogenic transformation.
The solution: We optimized the method of nonviral, CRISPR/Cas9 genome editing using large donor DNA delivery, knocked-in an anti-tumor single chain variable fragment (scFv) into the N-terminus of CD3ε and efficiently generated fusion protein (FP) T cells.
Key outcomes: These cells displayed FP integration within the TCR/CD3 complex, lower variability in gene expression compared to CAR-T cells and good cell expansion after transfection. CD3ε FP T cells were predominantly CD8+ effector memory T cells, and exhibited anti-tumor activity in vitro and in vivo. Dual targeting FP T cells were also generated through the incorporation of scFvs into other CD3 subunits and CD28. Compared to viral-based methods, this method serves as an alternative and versatile way of generating T cells with tumor-targeting receptors for cancer immunotherapy.
Published in Frontiers in Immunology (https://doi.org/10.3389/fimmu.2022.968395), Van To and colleagues review the current potential CAR-T cell targets for Cutaneous T cell lymphoma (CTCL), and the strategies required for more effective treatment of the disease. CTCL is a blood cancer caused by malignant T cells. While CAR-T cell therapy has had remarkable success against most types of blood cancers, its application to T cell cancers is limited.
The challenge: CAR-T cell therapies face a range of barriers against T cell cancers. These are associated with the creation and application of the therapy, as well as the cancer cell environment. CAR-T cells are engineered to identify a pre-determined target on cancer T cells. However, since both the malignant T cell and the therapy may share the target, CAR-T cells could kill healthy cells in the patient. Additionally, a shared target may also cause self-killing of the CAR-T cells.
Cancer cells can also reduce their expression of the target, which may lead to therapy resistance, and during the manufacture of CAR-T cells from patient blood, the therapy may become contaminated with cancerous cells.
The solutions: New therapeutic approaches are required as CTCL remains a difficult disease to treat, with low survival in patients, and limited curative options. Approaches to improve therapy barriers include selecting appropriate CAR-T cell targets, ideally with low expression on healthy cells and engineering CAR-T cells with multiple targets using different ‘logic-gate’ strategies tailored to strike the balance between the safety and efficacy of the therapy.
Published in Translational Oncology (https://doi.org/10.1016/j.tranon.2022.101477), Dr Anisha Suraiya and colleagues have demonstrated a novel CAR-T cell carrier system. CAR-T cell therapy aims to target and kill cancer cells, and boost a patient’s immune system to fight cancer. The use of CAR-T cells for the treatment of blood cancers has been revolutionary with unparalleled responses observed in some patients. Despite significant advances, the same positive impact in solid cancers, such as ovarian cancer, remains to be seen.
The challenge: Solid cancers are able to create an environment that supports cancer growth and ‘protects’ cancer cells from elimination. When CAR-T cells are exposed to this complex environment for an extended time their ability to kill cancer cells can be reduced.
The solution: Designing a delivery system that shields CAR-T cells from the impact of the cancer environment may improve outcomes of CAR-T therapy in solid cancers. To this end, Dr Suraiya and colleagues have investigated the use of gelatine-based microgels as CAR-T cell carriers.
Key outcomes: CAR-T cells are able to survive in the microgel carriers for up to seven days. Importantly, encapsulation in microgel carriers did not impact their ability to kill cancer cells. Indeed, CAR-T cells were found to be potent killers of 3D solid tumor cell culture models. This study provides evidence supporting the use of microgel as CAR-T delivery vehicles for the treatment of cancer.
Integrin-linked kinase (ILK) has been implicated as a molecular driver and mediator in both inflammation and tumorigenesis of the colon. However, a role for ILK in the tumor microenvironment (TME) and immune evasion has not been investigated. Here, we show a correlation of ILK expression with the immunosuppressive TME and cancer prognosis. We also uncover a role for ILK in the regulation of programmed death-ligand 1 (PD-L1) expression and immune cell cytotoxicity.
Cellular immunotherapy is revolutionizing cancer treatment. And off-the-shelf, or allogeneic, treatments are beginning to replace technologies developed individually for each patient.
This is good news because the potential of allogeneic therapies is great. They promise to significantly save time and cut costs compared to individualised, or autologous, treatments.
In Cells special issue ‘Allogeneic Cell Cancer Immunotherapies” editor Alan Trounson introduces five papers from leading research teams, including two from Cartherics’ scientists. They all tackle the major scientific hurdle the new technologies face: possible rejection of treatments by patients’ immune systems.
Trounson is impressed by the solutions presented. “Early progress is encouraging, but the outcome of human clinical trials remains essential to evaluate the safety and efficacy of these new allogeneic cell therapy approaches,” he notes.
Cellular immunotherapy is revolutionizing cancer treatment. However, autologous transplants are complex, costly, and limited by the number and quality of T cells that can be isolated from and expanded for re-infusion into each patient.
This paper demonstrates a stromal support cell-free in vitro method for the differentiation of T cells from umbilical cord blood hematopoietic stem cells (HSCs).
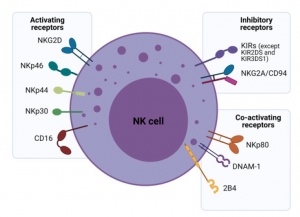
Natural killer (NK) cells are potent innate immune system effector lymphocytes armed with multiple mechanisms for killing cancer cells. Given the dynamic roles of NK cells in tumor surveillance, they are fast becoming a next-generation tool for adoptive immunotherapy. Many strategies are being employed to increase their number and improve their ability to overcome cancer resistance and the immunosuppressive tumor microenvironment.
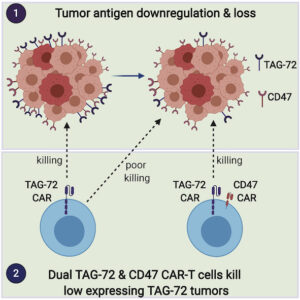
Chimeric antigen receptor (CAR) T cells have revolutionized blood cancer immunotherapy; however, their efficacy against solid tumors has been limited. A common mechanism of tumor escape from single target therapies is downregulation or mutational loss of the nominal epitope.
https://www.cell.com/molecular-therapy-family/oncolytics/fulltext/S2372-7705(21)00002-4
Despite progress in developing cell therapies, such as T cell or stemcell therapies to treat diseases, immunoincompatibility remains a major barrier to clinical application.
Given the fact that a host’s immune system may reject allogeneic transplanted cells, methods have been developed to genetically modify patients’primary cells.