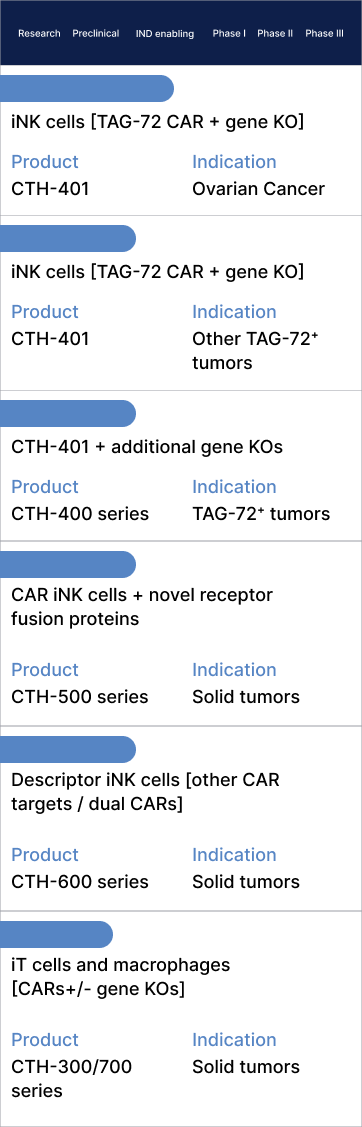
Product Pipeline
IND submission for lead clinical candidate, CTH-401, in 2026
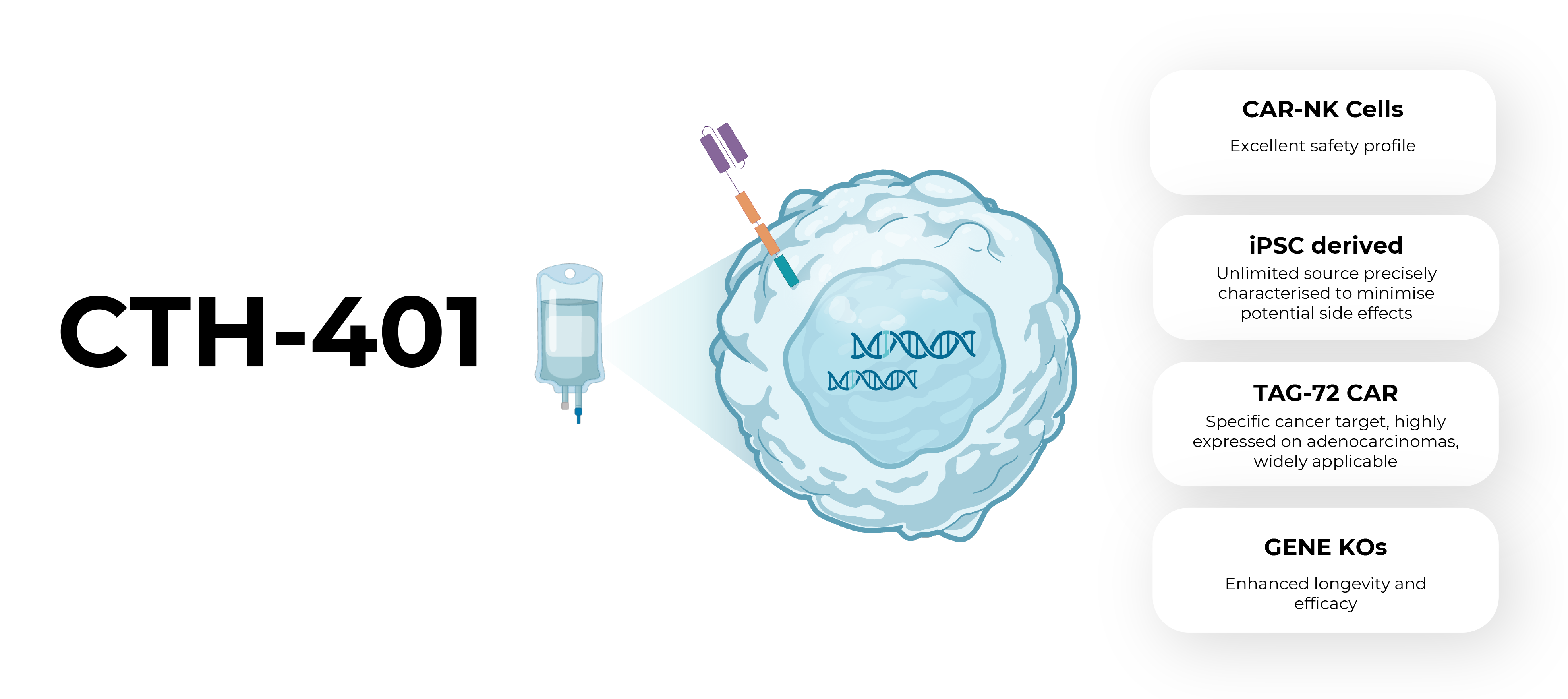
CTH-401 is an investigational allogeneic (“off-the-shelf”), induced pluripotent stem cell (iPSC)-derived, chimeric antigen receptor (CAR) natural killer (NK) cell therapy designed to target solid tumours. CTH-401 is genetically engineered to integrate a proprietary second-generation CAR construct that specifically targets tumour-associated glycoprotein-72 (TAG-72) and includes the deletion of two genes associated with immunosuppression. The TAG-72 CAR delivers precise on-target cytotoxic action (complementing the natural NK cell receptors that mediate tumour cell killing), while the gene deletions extend CTH-401’s survival and enhance its long-term efficacy. The final product is derived from a precisely characterized, clonal, iPSC master cell bank. Following a successful pre-investigational new drug (pre-IND) meeting with the US Food and Drug Administration (FDA), CTH-401 is planned to enter clinical trials for ovarian cancer in 2026. Subject to early clinical data, CTH-401 will subsequently enter a basket trial to target other TAG-72+ solid cancers.
R&D Pipeline – Allogeneic Products
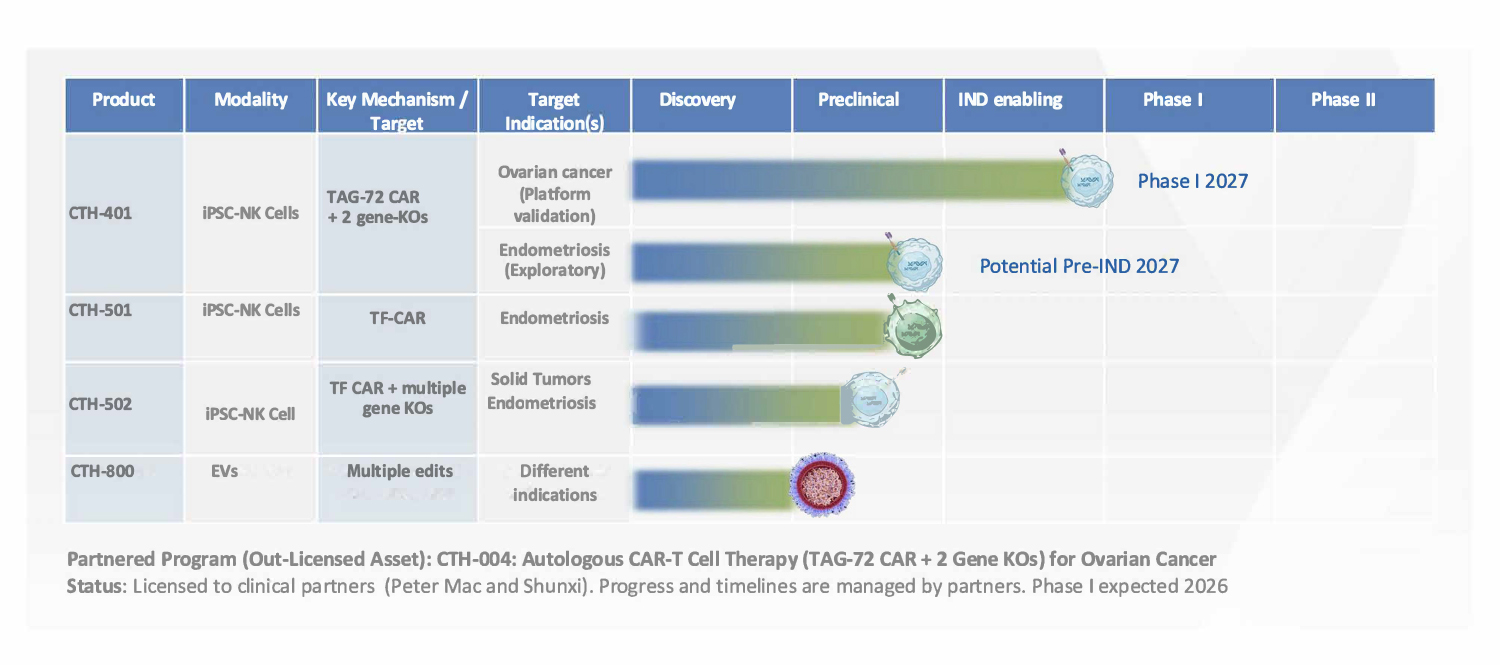
R&D Pipeline – Allogeneic iNK Cell Products