Cartherics technology consists of gene-editing iPSC, establishing stable clones, then deriving potentially limitless numbers of highly functional CAR-iNK cells for ‘off-the-shelf’ allogeneic cancer therapies.
The human immune system has evolved two distinct weapons to fight infection: antibodies and cell mediated immunity. Their combination would be an ideal weapon against cancer. But how to create this in a co-ordinated way? The advent of genetic engineering has enabled the fusion of these technologies and, thus, the ability to create Chimeric Antigen Receptors, or CARs.
CARs are engineered molecules that combine the antigen (e.g. cancer antigen) recognizing component of antibodies with cell activation signals. When the CARs are present at the surface of immune killer cells they enable these cells to not only recognise the cancer cells, but also kick-start the process of killing them.
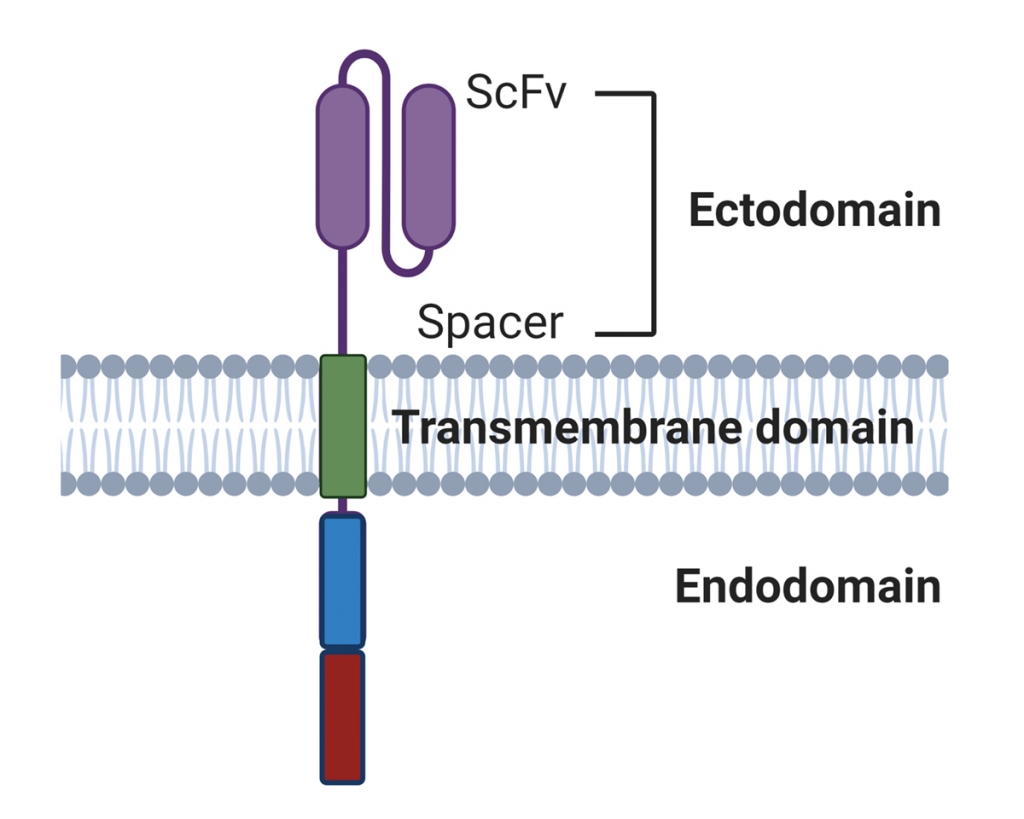
Figure 1. Basic design of a Chimeric Antigen Receptor. The scFv provides specificity for a nominal (cancer) antigen. The cytoplasmic endodomains provide activation signals for the cell. Second generation CAR’s (the most common) have two such signalling domains.
From: Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells 2021, 10, 1058. https://doi.org/ 10.3390/cells10051058
Cartherics is coupling CAR technology with techniques for editing out of strategic genes which normally act as hand brakes on immune responses. In healthy people these responses prevent immune overreactions, but when fighting cancer silencing them can enhance tumour cell killing. This multi-platform approach from Cartherics combines the best components of immune defences.
There are two types of killer cells which are commonly engineered to utilise CARs to target cancer: Natural Killer (NK) cells and T cells.
NK cells
- NK cells are rapidly emerging as the most important cellular weapon against cancer.
- They have a panel of natural surface receptors that can recognise abnormal cells, e.g. damaged, infected, stressed, aged or cancerous.
- If one cancer marker is lost, NK cells can still bind to others and kill the cell.
- They can be made more specific for certain cancers by insertion of a CAR.
- They don’t require activation before they can kill.
- They can be functionally enhanced by deletion of immune suppressing genes.
- They can be created in large numbers from pluripotent stem cells.
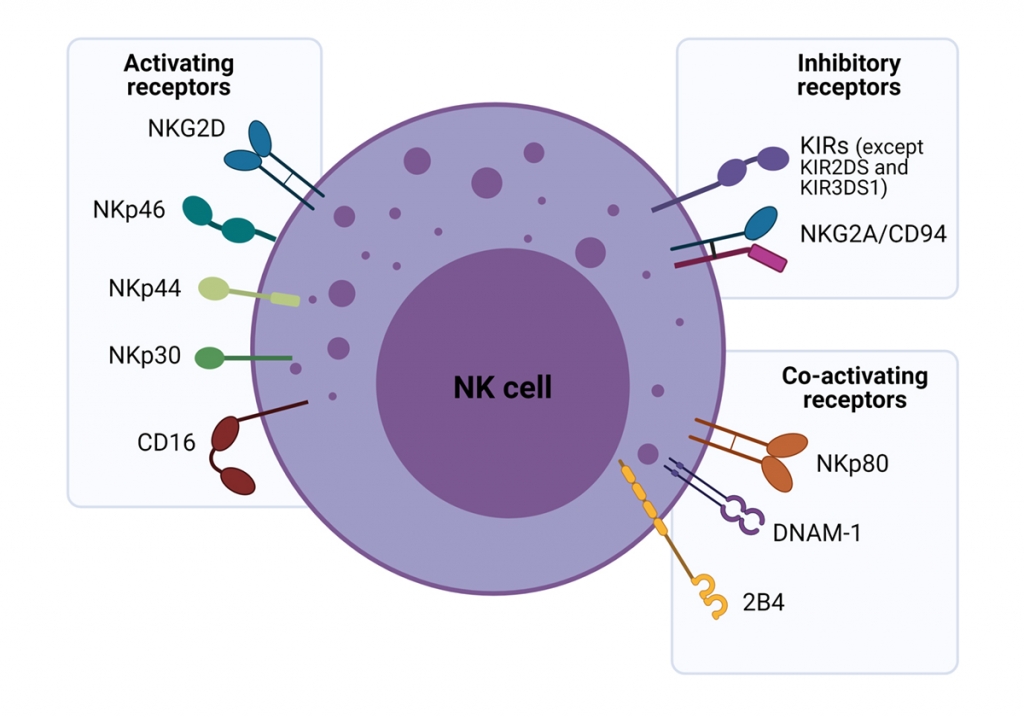
Figure 2. The major activating, co-activating and inhibitory receptors expressed on the surface of NK cells.
From: Islam, R.; Pupovac, A.; Evtimov, V.; Boyd, N.; Shu, R.; Boyd, R.; Trounson, A. Enhancing a Natural Killer: Modification of NK Cells for Cancer Immunotherapy. Cells 2021, 10, 1058. https://doi.org/ 10.3390/cells10051058
T cells
- T cells require activation before they kill. This also leads to their marked proliferation and long-lived retention as ‘memory’ cells which, being ‘pre-armed’, are able to respond rapidly to later challenge, e.g. return of the cancer.
- The problem with T cells is that they naturally only recognise one single determinant. If this is lost because, for example, the cancer has mutated, they are ineffective and the cancer rapidly expands.
- T cells can only recognize protein antigens. Antibodies can recognise any form of antigens such as sugars, carbohydrates, lipids, glycoproteins. Many cancer antigens are of these non-protein types.
- Additional cancer recognition can occur through insertion of CARs into the T cells.
- T cells are very difficult to generate in large numbers in vitro.
CAR-T cell technology has revolutionised blood cancer therapy with multiple products now approved by the FDA, including the following:
- ABECMA® (idecabtagene vicleucel) for relapsed or refractory multiple myeloma.
- BREYANZI® for relapsed or refractory large B-cell lymphoma and diffuse large B cell lymphoma.
- TECARTUSTM (brexucabtagene autoleucel) for refractory mantle cell lymphoma.
- KYMRIAHTM (tisagenlecleucel) for adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and young adult patients up to age 25 with relapsed or refractory acute lymphoblastic leukemia (ALL).
- YESCARTATM (axicabtagene ciloleucel) for adult patients with certain types of B-cell lymphoma.
Cartherics believes its novel CAR-T and CAR-NK cells have great potential for killing solid tumours like ovarian, gastric, prostate and breast cancer. The company is developing products for both solid and blood cancers.
CAR-killer cell products are of two basic types: autologous and allogeneic. The autologous therapies are generated from the patient’s own cells, while allogeneic or ‘off-the-shelf’ therapies use donor cells. There are pros and cons to each approach:
Autologous
Pros:
- Relatively lower regulatory requirements – no risk of rejection.
- Serve as proof-of-concept to test CAR safety and function.
Cons:
- Expensive (~$500K per treatment).
- Requires significant time (~4 weeks) to collect blood, then create and expand CAR-T cells in numbers sufficient for clinical treatment of cancer patients.
- Number and quality of CAR-T cells which can be produced from the cancer patient are limited as a result of prior lymphodepleting treatments such as chemotherapy and radiation.
Cartherics is exploring autologous CAR-T cell clinical trials in two diseases: Cutaneous T Cell Lymphoma (CTCL) and relapsed ovarian cancer. The company plans to undertake both trials under a development partnership with the Centre of Excellence in Cellular Immunotherapies at the Peter MacCallum Cancer Centre.
Allogeneic
Pros:
- Lower cost
- Healthier cells
- Readily available
Cons:
- Risk of immune rejection
- Donor T cells recognising patient as foreign, through the T Cell Receptor:
Graft versus host disease (GvHD); independent of CAR specificity. - Patient rejecting donor CAR-T cells.
- Donor T cells recognising patient as foreign, through the T Cell Receptor:
Cartherics’ Allogeneic CAR Platform
Cartherics is utilising a novel approach to develop ‘off-the-shelf’ CAR-NK and CAR-T cells.
- It uses its proprietary induced pluripotent stem cells (iPSC) which have limitless proliferative capacity.
- A key feature is deriving these iPSC from rare, triple homozygous HLA haplotype umbilical cord blood donor cells which are compatible for transplant to large numbers of patients. They won’t be rejected by the patient nor will the differentiated killer cells induce GvHD.
- Using proprietary technology, Cartherics is creating large numbers of clinically applicable CAR-iNK cells which also lack strategic immune suppression gene(s).
- Cartherics is also developing CAR-iT cells from the iPSC cells.
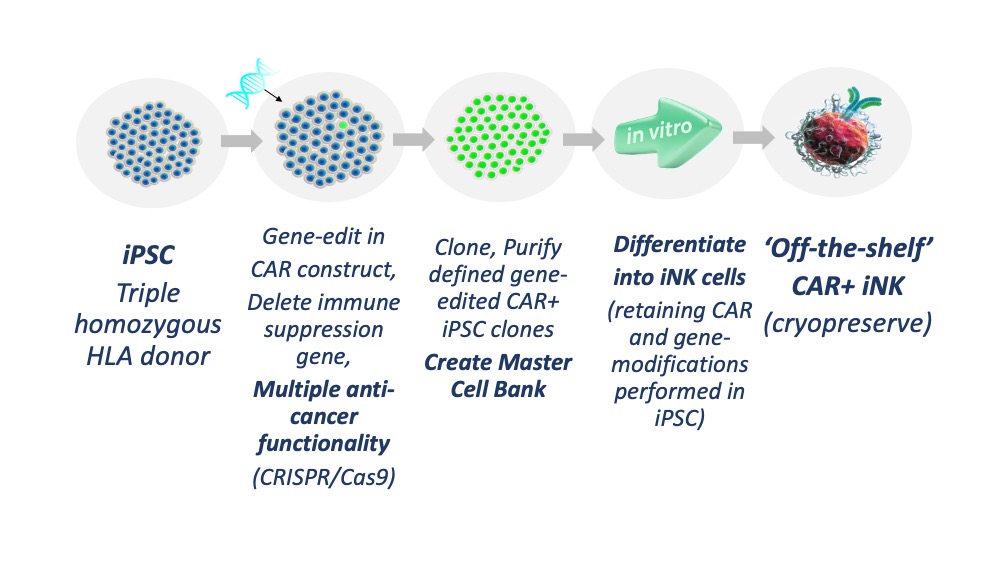
Figure 3. Cartherics strategy for creating gene-edited, CAR i-NK cells.
Courtesy of Nicholas Boyd
To advance the effectiveness of its CAR-iNK cells and CAR-iT cells, Cartherics is deleting key genes which act as hand brakes on immune cancer killing responses. Cartherics’ allogeneic platform will allow for:
- Unlimited supply of killer cells.
- Precisely defined product.
- ‘Off-the-shelf’ for on demand delivery to patients.
- Major reduction in manufacturing cost per treatment.
- Product with multiple anti-cancer modes of action.


